|
|
Some articles have not been moved to our new site yet.
As a result you have been redirected to our old site.
If you wish to return to our new site - click here.
Cannabinoids
and Multiple Sclerosis
Introduction
& Overview
Multiple
sclerosis is a disease of the brain and spinal cord
caused by demyelination (loss of the insulating sheath)
of nerve fibres, believed to be caused by some substance
dissolving or breaking-up fatty tissue of the nerve-sheath[1]. The condition is progressive but varies
in intensity, with remission of symptoms and relapse
commonly reported. Common symptoms include fatigue,
balance problems, muscle weakness, incontinence, muscle
spasms, pain and tremor.[2]
Current
treatments for MS are of little benefit, expensive,
and with risks of side effects. Alpha & º-interferon,
and corticosteroids have been found to have some value,
but symptoms are poorly-controlled by existing medications,
and no cure has been found. Many patients are unable
to tolerate the side-effects of conventional medication[3]
This
section reviews and describes the published scientific
evidence relating to the use of cannabis and cannabinoids
in the treatment of multiple sclerosis symptoms, and
the involvement of endocannabinoids in the development
of the MS disease process. Scientific developments in
this field are rapidly advancing, with an average of
one paper published every ten days over the past four
years since my last review of this field. Over that
period the results of many clinical trials have been
published, and the state of basic research into the
disease process has advanced dramatically.
Anecdotal
Evidence & Surveys
The
potential effect of cannabis on the symptoms of multiple
sclerosis were first reported by sufferers of the disease
introduced to cannabis by recreational or social users.
Such anecdotal evidence (i.e. not backed up at the time
by clinical assessment, animal or theoretical basis,
nor by clinical trials) includes case studies and self-completion
surveys.
Grinspoon[4] reports a number of anecdotal reports
of dramatic improvement in MS symptoms attributed to
marijuana (cannabis) use. Initially, these were unexpected
findings following social use of the drug. In one account,
Greg Paufler described a progressive degeneration, following
onset of MS in 1973, to bedridden status, and severe
side effects (dramatic weight gain, addiction to benzodiazepines)
from prescribed medicines. Following several social
åjoints" one evening, he astonished family and
friends by standing spontaneously for the first time
in months. He subsequently found that his symptoms deteriorated
without the drug, but improved dramatically during periods
when he was smoking cannabis. Grinspoon reviewed further
cases showing improvements in muscle spasms, tremor,
continence, ataxia (loss of muscle control) and insomnia.
Clare Hodges, an MS patient giving oral evidence to
the House of Lords enquiry, reported cannabis ågreatly
relieved" physical symptoms including discomfort
of bladder and spine, nausea and tremors, and stated
åCannabis helps my
body relax, I function and move much easier. The physical
effects are very clear, it is not just a vague feeling
of well-being."
In
a study of 112 MS patients self-medicating with cannabis
in the US and UK, Consroe et al[5] reported that 70% of more respondents
reported improvement in the following symptoms:
| |
Spasticity
at sleep onset
|
Pain
in muscles
|
| |
Spasticity
when awaking at night
|
Pain
in legs at night
|
| |
Tremor
(arms/head)
|
Depression
|
| |
Anxiety
|
Spasticity
when waking in morning
|
| |
Spasticity
when walking
|
Tingling
in face/arms/head/trunk
|
| |
Numbness
of chest/stomach
|
Pain
in face
|
| |
Weight
loss
|
Weakness
in legs
|
The authors considered these reports åstrongly
suggested cannabinoids may significantly relieve symptoms
of MS, particularly spasticity and pain",
and provided sufficient grounds for a properly controlled
clinical trial to test such claims objectively and conclusively.
A
German study[6] of 170 self-medicating cannabis users
found 11% of respondents reported using the drug successfully
in managing MS symptoms, the second most common use
behind depression, and concluded "this study demonstrates a successful use of cannabis
products for the treatment of a multitude of various
illnesses and symptoms. This use was usually accompanied
only by slight and in general acceptable side effects."
Mechoulam[7] reviews illegal use of
cannabis by MS patients. In a UK survey of 318 MS patients[8], 8% reported
using cannabis to relieve symptoms.
In
a survey of 780 MS patients in Canada, Page et al[9] found
"Forty-three percent had tried cannabis at some point
in their lives, 16% for medicinal purposes. Symptoms
reported to be ameliorated included anxiety/depression,
spasticity and chronic pain" and concluded
"Subjective improvements
in symptom experience were reported by the majority
of people with MS who currently use cannabis."
A survey of 131 patients with amytrophic lateral sclerosis[10], of which only 13 used cannabis, found
"cannabis may
be moderately effective at reducing symptoms of appetite
loss, depression, pain, spasticity, and drooling".
Simmons et al[11] studied responses to an internet survey
by 2529 respondents, finding cannabis commonly reported
as beneficial. Clarke et al[12] surveyed 220 MS patients in Canada,
finding "Medical
cannabis use was associated with male gender, tobacco
use, and recreational cannabis use. The symptoms reported
by medical cannabis users to be most effectively relieved
were stress, sleep, mood, stiffness/spasm, and pain."
Ware et al[13] reported
results of a survey of medicinal cannabis use in the
UK, noting "Medicinal cannabis use was reported by patients with
chronic pain (25%), multiple sclerosis and depression
(22% each), arthritis (21%) and neuropathy (19%). Medicinal
cannabis use was associated with younger age, male gender
and previous recreational use (p < 0.001)."
In
the Netherlands, cannabis has been available on prescription
from pharmacies since September 2003, Erkens et al[14] followed up 200 patients
prescribed the drug with a questionnaire survey, finding
"Cannabis was mainly used for chronic pain and muscle
cramp/stiffness.The indication of medicinal cannabis
use was in accordance with the labeled indications.
However, more than 80% of the patients still obtained
cannabis for medicinal purpose from the illegal circuit.
Because of the higher prices in pharmacies, ongoing
debate on the unproven effectiveness of the drug and
the hesitation by physicians to prescribe cannabis."
Animal
studies
Scientists
have developed animal models for MS in rats, mice and
guinea-pigs in the form of an experimental autoimmune
encephalomyelitis (EAE). In guinea-pigs, Lyman et al[15] found 98% of animals treated with placebo
died, whereas 95% of THC-treated animals survived the
disease process, with much reduced inflammation of brain
tissue. In rats, Wirguin et al[16]
found ∆8THC
significantly reduced neurological deficits in two strains
of EAE inoculated rats.
Baker
et al[17], studying tremor and spasticity in mice,
concluded: "The
exacerbation of these signs after antagonism of the
CB1 and CB2 receptors, notably the CB1 receptor... indicate
that the endogenous cannabinoid system may be tonically
active in the control of tremor and spasticity. This
provides a rationale for patients' indications of the
therapeutic potential of cannabis in the control of
the symptoms of multiple sclerosis, and provides a means
of evaluating more selective cannabinoids in the future."
Achiron et al[18]
studying rats, found reduction in the inflammatory response
in the brain and spinal cord in animals treated with
dexanabinol, a synthetic cannabinoid, and concluded
"dexanabinol
may provide an alternative mode of treatment for acute
exacerbations of multiple sclerosis (MS)".
Pop[19] reviews the development of dexanabinol,
a non-psychoactive cannabinoid and NMDA antagonist developed
by "Pharmos Corp for the potential treatment of cerebral
ischemia... and multiple sclerosis (MS)"
commenting "A Notice of Allowance was received in March 1999 on
a patent covering the use of the drug in the treatment
of MS [324163]. The use of dexanabinol and its derivatives
to treat MS is described in US-05932610 [358503]."
Fernandez-Ruiz[20] noted "Data,
initially anecdotal, but recently supported on more
solid experimental evidence, suggest that cannabinoids
might be beneficial in the treatment of some of the
symptoms of multiple sclerosis (MS). Despite this evidence,
there are no data on the possible changes in cannabinoid
CB(1) or CB(2) receptors, the main molecular targets
for the action of cannabinoids, either in the postmortem
brain of patients with MS or in animal models of this
disease (EAE)", concluding "generation
of EAE in Lewis rats would be associated with changes
in CB(1) receptors in striatal and cortical neurons,
which might be related to the alleviation of some motor
signs observed after the treatment with cannabinoid
receptor agonists in similar models of MS"
Baker et al[21] found "In
areas associated with nerve damage, increased levels
of the endocannabinoids... were detected"
and concluded "These
studies provide definitive evidence for the tonic control
of spasticity by the endocannabinoid system and open
new horizons to therapy of multiple sclerosis, and other
neuromuscular diseases, based on agents modulating endocannabinoid
levels and action, which exhibit little psychotropic
activity."
Recent
studies of the biological basis of MS largely confirm
the efficacy of cannabinoids in relief of muscle spasticity,
including the endogenous cannabinoids amandamine and
2-arachidonoyl glycerol[22], and
by dexanabinol via non-receptor mediated reduction of
inflammation[23].
Baker at al[24] considered research to have demonstrated
"definitive evidence
for the tonic control of spasticity by the endocannabinoid
system". In a series of reviews of the
implications of recent fundamental cannabinoid research
on therapeutic potential, Pertwee[25][26] stated:
"...uses for
CB1 receptor agonists include the suppression of muscle
spasm/spasticity associated with multiple sclerosis...".
The main active constituent of cannabis - THC is one
such CB1 receptor agonist.
Killistein
et al[27] concluded "endogenous
cannabinoids appear to play an important role in signal
transduction, which may be starting points for therapy
regarding... multiple sclerosis" Klein
et al[28] reported "The
effect of cannabimimetic agents on the function of immune
cells such as T and B lymphocytes, natural killer cells
and macrophages has been extensively studied over the
past several decades using human and animal paradigms
involving whole animal models as well as tissue culture
systems. From this work, it can be concluded that these
drugs have subtle yet complex effects on immune cell
function and that some of the drug activity is mediated
by cannabinoid receptors expressed on the various immune
cell subtypes... Further studies will define the precise
structure and function of the putative immunocannabinoid
system, the potential therapeutic usefulness of these
drugs in chronic diseases such as acquired immune deficiency
syndrome and multiple sclerosis" Lambert
et al[29], studying N-palmitoylethanolamine (PEA),
an analogue of anandamide, found "PEA
is accumulated during inflammation and has been demonstrated
to have a number of anti-inflammatory effects... It
is now engaged in phase II clinical development, and
two studies regarding the treatment of chronic lumbosciatalgia
and multiple sclerosis are in progress."
Brooks et al reported "Activation of cannabinoid receptors causes inhibition
of spasticity, in a mouse model of multiple sclerosis",
finding that Arvanil, a "structural
"hybrid" between capsaicin and anandamide,
was a potent inhibitor of spasticity at doses (e.g.
0.01 mg/kg i.v.) where capsaicin and cannabinoid CB(1)
receptor agonists were ineffective"
Wilkinson
et al[30], studying a mouse model of MS, found
"Whilst (cannabis
extract) inhibited spasticity in the mouse model of
MS to a comparable level, it caused a more rapid onset
of muscle relaxation, and a reduction in the time to
maximum effect compared with Delta9THC alone. The Delta9THC-free
extract or cannabidiol (CBD) caused no inhibition of
spasticity" concluding re antispasticity
"Delta9THC was the active constituent, which might
be modified by the presence of other components"
Ni et al[31]
noted "Cannabinoid
receptor agonists have been shown to downregulate immune
responses and there is preliminary evidence that they
may slow the progress of MS." Raman
et al[32] found
"Administration
at the onset of tremors delayed motor impairment and
prolonged survival in Delta(9)-THC treated mice when
compared to vehicle controls" Mestre
et al[33] concluded
their results to suggest "manipulation
of the endocannabinoid system as a possible strategy
to develop future MS therapeutic drugs"
Weydt et al[34] noted the effect on ALS-induced
mice of non-psychoactive CBN (cannabinoid) "significantly
delays disease onset by more than two weeks while survival
was not affected"
Ortega-Gutierrez
et al[35] found that in mice, an anandamide reuptake
inhibitor UCM707 would "reduce
microglial activation, diminish major histocompatibility
complex class II antigen expression, and decrease cellular
infiltrates in the spinal cord. Additionally, in microglial
cells, UCM707 decreases the production of the proinflammatory
cytokines tumor necrosis factor (TNF)-alpha, interleukin
(IL)-1beta, and IL-6; reduces nitric oxide levels and
inducible nitric oxide synthase expression; and is able
to potentiate the action of a subeffective dose of the
endocannabinoid anandamide. Overall, these results suggest
that agents able to activate the endocannabinoid system
could constitute a new series of drugs for the treatment
of MS." De Lago et al[36]
found "UCM707,
like other endocannabinoid uptake inhibitors reported
previously, significantly reduced spasticity of the
hindlimbs in a chronic relapsing EAE mice, a chronic
model of MS."
Studying
mice, Arevalo-Martin et al[37] found "cannabinoids reduced microglial activation, abrogated
major histocompatibility complex class II antigen expression,
and decreased the number of CD4+ infiltrating T cells
in the spinal cord. Both recovery of motor function
and diminution of inflammation paralleled extensive
remyelination" and concluded there were
"potential therapeutic
implications in demyelinating pathologies such as MS;
in particular, the possible involvement of cannabinoid
receptor CB2 would enable nonpsychoactive therapy suitable
for long-term use." Croxford & Miller[38] found "cannabinoids
are useful for symptomatic treatment of spasticity and
tremor in chronic-relapsing experimental autoimmune
encephalomyelitis. Cannabinoids, however, have reported
immunosuppressive properties. We show that the cannabinoid
receptor agonist, R+WIN55,212, ameliorates progression
of clinical disease symptoms in mice with preexisting
TMEV-IDD"
In
rat model of MS (CREAE), Cabranes et al[39] reported
"CB(1) receptors
were affected by the development of CREAE in mice exhibiting
always down-regulatory responses that were circumscribed
to motor-related regions and that were generally more
marked during the acute and chronic phases. These observations
may explain the efficacy of cannabinoid agonists to
improve motor symptoms (spasticity, tremor, ataxia)
typical of MS in both humans and animal models."
Human
studies
Several
researchers[40][41][42] have
commented upon the difficulties involved in conducting
proper research on the effects of cannabinoids on medical
conditions, including MS, in the light of the legal
status of cannabis. Robson[43] comments "the methodological challenges to human research involving
a pariah drug are formidable"
Petro
& Ellenberger[44], in a small double-blind clinical trial
found 10mg THC significantly (p<.01) reduced spasticity
in patients with MS or similar conditions, compared
to placebo. In an earlier double-blind crossover trial,
Ungerleider et al[45] reported åAt
doses greater than 7.5 mg there was significant improvement
in patient ratings of spasticity compared to placebo.
These positive findings in a treatment failure population
suggest a role for THC in the treatment of spasticity
in multiple sclerosis." Clifford[46], in a trial involving
8 patients severely disabled with tremor and ataxia,
reported significant improvement in two patients. Case
study reports[47][48]
suggest that cannabis can suppress pendular nystagmus
(jerky eye movements) in patients with multiple sclerosis.
In
a pilot study involving two patients, Brenneison et
al[49]
reported "Oral
and rectal THC reduced at a progressive stage of illness
the spasticity, rigidity, and pain, resulting in improved
active and passive mobility." In a single
case double-blind trial, Maurer et al[50] found THC "showed
a significant beneficial effect on spasticity. In the
dosage of THC used no altered consciousness occurred."
Consroe et al[51]
reported cannabidiol (CBD) to produce dose-related improvements
in dystonic movement disorders. Malec et al[52] found spinal cord injured persons reported
decreased spasticity with marijuana use. Other papers
have also reported potential benefits of cannabinoids,
including crude marijuana[53][54],
and the synthetic Nabilone[55], where Martyn et al found clear improvement
in well-being, reduced pain from muscle spasm, and reduced
frequency of nocturia during the treatment condition
(1mg Nabilone every other day) compared to worsening
of symptoms during no treatment or placebo conditions.
Meinck
et al[56] reported "The
chronic motor handicaps of a 30-year-old multiple sclerosis
patient acutely improved while he smoked a marihuana
cigarette. This effect was quantitatively assessed by
means of clinical rating, electromyographic investigation
of the leg flexor reflexes and electromagnetic recording
of the hand action tremor. It is concluded that cannabinoids
may have powerful beneficial effects on both spasticity
and ataxia that warrant further evaluation."
The improvements in tremor reported by Meinck &
Clifford are dramatically demonstrated in fig 4 below.
Fig
4 - Effects of THC on tremor in MS patients
Clifford
(1983)
Meinck et al (1989)
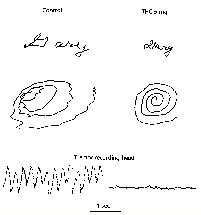 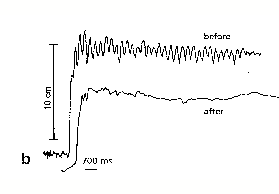 In
a small-scale Dutch trial on MS patients with severe
spasticity, Killestein et al[57] noted "Both
drugs were safe, but adverse events were more common
with plant-extract treatment. Compared with placebo,
neither THC nor plant-extract treatment reduced spasticity.
Both THC and plant-extract treatment worsened the participant's
global impression.", prompting Thompson
& Baker[58]
to conclude that the drug was potentially useful but
not yet ready for widespread clinical use. Smith[59], whilst accepting the cannabinoids effectivelyreduce
pain and spasticity in multiple sclerosis, highlighted
the limited evidence from available trials, and questioned
whether they are superior to conventional medications.
"Whether or not
cannabinoids do have therapeutic potential in the treatment
of MS, a further issue will be whether synthetic cannabinoids
should be used in preference to cannabis itself. Smoking
cannabis is associated with significant risks of lung
cancer and other respiratory dysfunction. Furthermore,
delta9-THC, as a broad-spectrum cannabinoid receptor
agonist, will activate both CB1 and CB2 receptors. Synthetic
cannabinoids, which target specific cannabinoid receptor
subtypes in specific parts of the CNS, are likely to
be of more therapeutic use than delta9-THC itself. If
rapid absorption is necessary, such synthetic drugs
could be delivered via aerosol formulations."
Fernandez[60] recognised the scientific basis and
called for more extensive and long-term clinical trials.
Clark[61] called
for reclassification of cannabis in the USA to allow
physicians to prescribe marijuana for MS. Williamson
& Evans[62] conducted
a wide ranging review into the therapeutic uses of cannabinoids,
commenting "Cannabis
is frequently used by patients with multiple sclerosis
(MS) for muscle spasm and pain, and in an experimental
model of MS low doses of cannabinoids alleviated tremor.
Most of the controlled studies have been carried out
with THC rather than cannabis herb and so do not mimic
the usual clinical situation." Mechoulam[63]
noted "Clinical
work in multiple sclerosis, which may lead to the approval
of tetrahydrocannabinol as a drug for this condition"
Schlicker et al[64] noted "Cannabis (marijuana)... has the potential for the
development of useful agents for the treatment of ...
multiple sclerosis." Carter & Rosen[65] reported "Marijuana
is a substance with many properties that may be applicable
to the management of amyotrophic lateral sclerosis (ALS).
These include analgesia, muscle relaxation, bronchodilation,
saliva reduction, appetite stimulation, and sleep induction."
Guzman et al[66] concluded
"The neuroprotective
effect of cannabinoids may have potential clinical relevance
for the treatment of neurodegenerative disorders such
as multiple sclerosis"
Cannabinoids
and Muscle Spasticity
In
1981 Petro & Ellenberger[67] noted "Spasticity is a common neurologic condition in patients
with multiple sclerosis, stroke, cerebral palsy or an
injured spinal cord. Animal studies suggest that THC
has an inhibitory effect on polysynaptic reflexes. Some
spastic patients claim improvement after inhaling cannabis"
and, in a mixed patient group, found "10
mg THC significantly reduced spasticity by clinical
measurement (P < 0.01)." In spinal
injury patients, Malec et al[68] found "spinal cord injured persons reported decreased spasticity
with marijuana use". The BMA report
recommended 'carefully controlled trials of cannabinoids in patients
with chronic spastic disorders which have not responded
to other drugs'
Pertwee[69] reported in 2002 "There is
a growing amount of evidence to suggest that cannabis
and individual cannabinoids may be effective in suppressing
certain symptoms of multiple sclerosis and spinal cord
injury, including spasticity and pain.",
noting "Clinical
... trials have shown that cannabis, Delta(9)-tetrahydrocannabinol,
and nabilone can produce objective and/or subjective
relief from spasticity, pain, tremor, and nocturia in
patients with multiple sclerosis (8 trials) or spinal
cord injury (1 trial)." Pertwee &
Ross[70] noted "released
endocannabinoids mediate reductions both in inflammatory
pain and in the spasticity and tremor of multiple sclerosis".
Brooks et al[71]
reported "Activation
of cannabinoid receptors causes inhibition of spasticity"
Similarly,
Smith[72] noted "There
is a large amount of evidence to support the view that
the psychoactive ingredient in cannabis, delta9-tetrahydrocannabinol
(delta9-THC), and cannabinoids in general, can reduce
muscle spasticity and pain under some circumstances.
Cannabinoid (CB1) receptors in the CNS appear to mediate
both of these effects and endogenous cannabinoids may
fulfil these functions to some extent under normal circumstances"
while cautioning "it is still questionable whether cannabinoids are
superior to existing, conventional medications for the
treatment of spasticity and pain. In the case of spasticity,
there are too few controlled clinical trials to draw
any reliable conclusion at this stage."
In a 2001 review, Kalant[73] noted "Recent
evidence clearly demonstrates analgesic and anti-spasticity
effects that will probably prove to be clinically useful."
A
small Dutch clinical trial[74] found "Compared
with placebo, neither THC nor (cannabis) plant-extract
treatment reduced spasticity", prompting
Thomas & Baker[75] to question
whether western medicine was ready for cannabinoid therapy.
However, a larger scale trial of MS patients by GW Pharmaceuticals[76] reported
"THC:CBD medicine
provided a highly statistically significant improvement
in the symptom of spasticity".
There
is a large amount of research recently undertaken or
underway into the antispastic effects of cannabinoids,
particularly in patients with multiple sclerosis or
spinal injury. Less research effort has been conducted
to date into patients with spasticity arising from other
neurological conditions, although animal studies suggest
the anti-spastic effects of CB1 receptor agonists are
not confined to these conditions.
GW
Pharmaceuticals & Clinical Trials
In
November 2002, GW Pharmaceuticals reported the results
of Phase III clinical trials of cannabis-extract based
medicines on MS patients[77],
finding:
(a)
"In a double-blind parallel group study comparing
the efficacy of GW"s THC:CBD product with placebo
in the treatment of neuropathic pain in 66 patients
with MS, the THC:CBD medicine provided highly statistically
significant relief of pain in comparison with placebo
and highly statistically significant reduction in sleep
disturbance."
(b)
"In a double-blind parallel group study comparing
the efficacy of GW"s THC:CBD product with placebo
in the treatment of chronic refractory pain in 70 patients
with MS and other neurological conditions, the THC:CBD
medicine provided statistically significant pain relief
(as evidenced by the diminished use of analgesic rescue
medication) and statistically significant reduction
in sleep disturbance."
(c)
"In a double-blind parallel group study comparing
the efficacy of GW"s THC:CBD product with placebo
in the treatment of a number of symptoms in 160 patients
with MS, the THC:CBD medicine provided a highly statistically
significant improvement in the symptom of spasticity.
Positive trends were also observed in a number of other
MS symptoms (providing useful additional support to
significant results obtained in Phase II trials)."
Dr Philip Robson, GW Medical Director, commented: "These
rigorous randomised placebo-controlled trials indicate
that GW"s cannabis-based medicine can provide additional
benefits over and above that of standard treatments
in these serious and refractory neurological conditions.
The results show statistically significant reductions
in neuropathic pain, which is recognised as being difficult
to treat and is often particularly distressing. There
were also significant improvements in other symptoms
in patients with MS, notably spasticity and sleep disturbance.
In my opinion, it is this broad spectrum of activity,
coupled with an excellent safety profile, which gives
GW"s cannabis-based medicine the potential to make
a unique contribution towards improving the quality
of life of patients with these chronic disabling diseases."
Svendsen
et al[78] conducted a clinical trial in Denmark
using Dronabinol (synthetic THC) finding "Median
spontaneous pain intensity was significantly lower during
dronabinol treatment than during placebo treatment...
On the SF-36 quality of life scale, the two items bodily
pain and mental health indicated benefits from active
treatment compared with placebo", the
same team[79] later
reported "Dronabinol
reduced the spontaneous pain intensity significantly
compared with placebo" In a clinical
trial of cannabis extracts in Switzerland, Vaney et
al[80] noted
"trends in favour
of active treatment were seen for spasm frequency, mobility
and getting to sleep. In the 37 patients (per-protocol
set) who received at least 90% of their prescribed dose,
improvements in spasm frequency (P = 0.013) and mobility
after excluding a patient who fell and stopped walking
were seen (P = 0.01)" and concluded
"A standardized
Cannabis sativa plant extract might lower spasm frequency
and increase mobility with tolerable side effects in
MS patients with persistent spasticity not responding
to other drugs."
A
clinical trial of cannabis extracts on bladder function
in MS patients by Brady et al[81] found "Urinary
urgency, the number and volume of incontinence episodes,
frequency and nocturia all decreased significantly following
treatment (P <0.05, Wilcoxon's signed rank test).
However, daily total voided, catheterized and urinary
incontinence pad weights also decreased significantly
on both extracts. Patient self-assessment of pain, spasticity
and quality of sleep improved significantly (P <0.05,
Wilcoxon's signed rank test) with pain improvement continuing
up to median of 35 weeks. There were few troublesome
side effects" In a large-scale (n=630)
clinical trial of cannabis extract, THC and placebo
on bladder function Freeman et al[82] found "All
three groups showed a significant reduction, p<0.01,
in adjusted episode rate (i.e. correcting for baseline
imbalance) from baseline to the end of treatment: cannabis
extract, 38%; THC, 33%; and placebo, 18%. Both active
treatments showed significant effects over placebo (cannabis
extract, p=0.005; THC, p=0.039). Conclusion: The findings
are suggestive of a clinical effect of cannabis on incontinence
episodes in patients with MS."
A
clinical trial of Sativex on MS symptoms including spasticity,
spasms, bladder problems, tremor or pain by Wade et
al[83] reported "Following CBME the primary symptom score reduced from
mean (SE) 74.36 (11.1) to 48.89 (22.0) following CBME
and from 74.31 (12.5) to 54.79 (26.3) following placebo
[ns]. Spasticity VAS scores were significantly reduced
by CBME (Sativex) in comparison with placebo (P =0.001)."
A clinical trial of 1:1 THC/CBD Sativex on 66x MS patients
suffering central pain by Rog et al[84] found "(Sativex)
was superior to placebo in reducing the mean intensity
of pain (CBM mean change -2.7, 95% CI: -3.4 to -2.0,
placebo -1.4 95% CI: -2.0 to -0.8, comparison between
groups, p = 0.005) and sleep disturbance (CBM mean change
-2.5, 95% CI: -3.4 to -1.7, placebo -0.8, 95% CI: -1.5
to -0.1, comparison between groups, p = 0.003). CBM
was generally well tolerated, although more patients
on CBM than placebo reported dizziness, dry mouth, and
somnolence"
Reviewing
clinical trial evidence in 2005, Teare & Zajicek[85]
noted "Recent
clinical studies to treat symptoms of multiple sclerosis
have shown varying results, which may reflect issues
relating to the way in which such studies were conducted.
There is now increasing interest in the potential role
of cannabinoids not only in symptom relief, but also
for their possible neuroprotective actions."
Zajicek et al[86] followed up clinical trial patients
invited to continue treatment with THC, plant extract
or placebo for 12 months, finding a "small
treatment effect on muscle spasticity as measured by
change in Ashworth score from baseline to 12 months"
for THC (1.82) and extract (0.1) compared to placebo
(-0.23), and concluded "These data provide limited evidence for a longer term
treatment effect of cannabinoids. A long term placebo
controlled study is now needed to establish whether
cannabinoids may have a role beyond symptom amelioration
in MS." In a clinical review, Azad &
Rammes[87]
concluded "In
multiple sclerosis, cannabinoids have been shown to
have beneficial effects on spasticity, pain, tremor
and bladder dysfunction."
Solaro[88] conducted two clinical trials of Dronabinol
and Sativex on pain in MS patients, finding "Active
drugs were superior to placebo in reducing the mean
intensity of pain, although patients reported side effects
such as dizziness and somnolence more frequently"
In a clinical trial of sublingual extracts, Wade et
al[89] found "Pain
relief associated with both THC and CBD was significantly
superior to placebo. Impaired bladder control, muscle
spasms and spasticity were improved by CME in some patients
with these symptoms." In a clinical
trial studying effects of cannabinoids on tremor in
MS patients, Fox et al[90] found
"no significant
improvement in any of the objective measures of upper
limb tremor with cannabis extract compared to placebo.
Finger tapping was faster on placebo compared to cannabis
extract (p < 0.02). However, there was a nonsignificant
trend for patients to experience more subjective relief
from their tremors while on cannabis extract compared
to placebo" In a further clinical trial,
Notcutt et al[91]
studied the effect of extracts on relief of chronic
pain, finding "Extracts which contained THC proved most effective
in symptom control."
GW
Pharmaceuticals[92] reported interim results of clinical
trials in 2003, including "THC:CBD
(narrow ratio) caused statistically significant reductions
in neuropathic pain in patients with MS and other conditions.
In addition, improvements in other MS symptoms were
observed as well" and concluding "The phase II trials provided positive results and
confirmed an excellent safety profile for cannabis-based
medicines"
Reviewing
results of clinical trials, Corey[93] noted
"Two large trials
found that cannabinoids were significantly better than
placebo in managing spasticity in multiple sclerosis.
Patients self-reported greater sense of motor improvement
in multiple sclerosis than could be confirmed objectively.
In smaller qualifying trials, cannabinoids produced
significant objective improvement of tics in Tourette's
disease, and neuropathic pain. A new, non-psychotropic
cannabinoid also has analgesic activity in neuropathic
pain." Reviewing results of early clinical
trials in 2004, Smith[94] conceded "results
of preclinical trials also lend support to the hypothesis
that the endogenous cannabinoid system may be involved
in the regulation of spasticity and pain"
Fernandez-Ruiz et al[95] concluded
"the control
of movement is one of the more relevant physiological
roles of the endocannabinoid transmission in the brain"
Shakespeare
et al[96] criticised studies of spasticity which
failed to use the standardised åAshworth" scale
to score results. In a controlled clinical trial Zajicek
et al[97] found "Treatment with cannabinoids did not have a beneficial
effect on spasticity when assessed with the Ashworth
scale. However, though there was a degree of unmasking
among the patients in the active treatment groups, objective
improvement in mobility and patients' opinion of an
improvement in pain suggest cannabinoids might be clinically
useful." Killestein et al[98] conducted
a clinical trial of oral THC and cannabis plant extract
in 16 MS patients, finding both drugs to be safe but
neither to be effective at reducing spasticity, and
both åworsened the
participant"s global perception".
However, another paper from the same study[99] noted "The
results suggest pro-inflammatory disease-modifying potential
of cannabinoids in MS" Killestein et
al[100] concluded in 2004 that "convincing evidence that cannabinoids are effective
in MS is still lacking" and that "it
is also not possible to conclude definitely that cannabinoids
are ineffective"[101]
Russo
& Guy[102], of GW Pharmaceuticals, reported in
2006 "CBD is
demonstrated to antagonise some undesirable effects
of THC including intoxication, sedation and tachycardia,
while contributing analgesic, anti-emetic, and anti-carcinogenic
properties in its own right. In modern clinical trials,
this has permitted the administration of higher doses
of THC, providing evidence for clinical efficacy and
safety for cannabis based extracts in treatment of spasticity,
central pain and lower urinary tract symptoms in multiple
sclerosis, as well as sleep disturbances, (and) peripheral
neuropathic pain... The hypothesis that the combination
of THC and CBD increases clinical efficacy while reducing
adverse events is supported." Perez[103] reviewed the results of clinical trials
of Sativex, concluding "Clinical
assessment of this combined cannabinoid medicine has
demonstrated efficacy in patients with intractable pain
(chronic neuropathic pain, pain due to brachial plexus
nerve injury, allodynic peripheral neuropathic pain
and advanced cancer pain), rheumatoid arthritis and
multiple sclerosis (bladder problems, spasticity and
central pain), with no significant intoxication-like
symptoms, tolerance or withdrawal syndrome"
Treating
the disease process?
Increasing
evidence is emerging from receptor and animal studies
that cannabinoids may be involved in the degenerative
disease process involved in the development of MS, and
that cannabinoid therapy may offer hope of halting or
even reversing the disease process.
Molina-Holgado
et al[104] found anandamide (endogenous CB1 cannabinoid
receptor agonist) reduced the effects of encephalomyelitis
in mice, suggesting a receptor-mediated mode of action
in arresting or reducing the autoimmune response considered
to be involved in the MS disease process.
Van
Oosten et al[105] reported a case study of a 46 year
old woman treated for obesity with a cannabinoid-receptor
antagonist who developed MS some months later.
Jackson
et al[106] studied genetically-modified mice where
the CB1 receptors had been disabled, noting an increase
in demyelination responses, and concluding that the
results "strengthen
the hypothesis of neuroprotection elicited via cannabinoid
receptor 1 signaling." Pryce et al[107], in a study on neurodegeneration in
mice, concluded "in
addition to symptom management, cannabis may also slow
the neurodegenerative processes that ultimately lead
to chronic disability in multiple sclerosis"
In
rats, Carrier et al[108] noted "2-AG
activation of CB(2) receptors may contribute to the
proliferative response of microglial cells, as occurs
in neurodegenerative disorders" Kim
et al[109] reported "AM1241
is a cannabinoid CB2 receptor selective agonist that
has been shown to be effective in models of inflammation
and hyperalgesia... treatment with AM1241 was effective
at slowing signs of disease progression when administered
after onset of signs in an ALS mouse model (hSOD1(G93A)
transgenic mice). Administration at the onset of tremors
delayed motor impairment in treated mice when compared
to vehicle controls. Three conditions of ALS, the loss
of motor function, paralysis scoring and weight loss,
were analyzed using a mathematical model. Loss of motor
function (as assessed by performance on a rotarod) was
delayed by 12.5 days in male mice by AM1241. In female
mice, AM1241 extended rotarod performance by 3 days,
although this was not statistically significant. In
male mice, AM1241 also extended by 5 days the time to
reach the 50% point on a visually-assessed performance
scale."
Stella[110] investigated the effect of cannabinoids
on glial cells involved in the MS disease process, noting
"Recent evidence suggests that glial cells also express
components of the cannabinoid signaling system and marijuana-derived
compounds act at CB receptors expressed by glial cells,
affecting their functions" Following
a tissue-culture study showing that JWH-015 - a CB2
agonist, reduced neurodegenerative activity in microglial
cells, Ehrhart et al[111] postulated "beneficial
effects provided by cannabinoid receptor CB2 modulation
in neurodegenerative diseases" Eljaschewitsch
et al[112]
found "the endocannabinoid
anandamide (AEA) protects neurons from inflammatory
damage by CB(1/2) receptor-mediated rapid induction
of mitogen-activated protein kinase phosphatase-1 (MKP-1)
in microglial cells" Tagliaferro et
al[113] reported "long-term neuroprotective
effects observed after cannabinoid treatments"
Fujiwara
et al[114] reported "Delta9-THC
markedly inhibited the neurodegeneration in experimental
allergic encephalomyelitis (EAE), an animal model of
multiple sclerosis and reduced the elevated glutamate
level of cerebrospinal fluid induced by EAE. These therapeutic
effects on EAE were reversed by SR141716A"
Yang et al[115] reported "ameliorating
effects of cannabinoids on axonal injury associated
with multiple sclerosis are achieved by its direct action"
Reviewing neurodegenerative studies, Yiangou et al[116] concluded
"CB2 specific
agonists deserve evaluation in the progression of MS
and ALS" Witting et al[117]
reported "activation
of cannabinoid receptors reduces the production and
diffusion of harmful mediators" Bilsland
et al[118] concluded "cannabinoids
have significant neuroprotective effects"
Jackson
et al[119] concluded "neuroprotection
could be elicited through the cannabinoid receptor 1,
and point towards a potential therapeutic role for cannabinoid
compounds in demyelinating conditions such as multiple
sclerosis", further concluding[120] "There
is increasing evidence for cannabinoid-mediated control
of symptoms, which is being more supported by the underlying
biology. However there is accumulating evidence in vitro
and in vivo to support the hypothesis that the cannabinoid
system can limit the neurodegenerative possesses that
drive progressive disease, and may provide a new avenue
for disease control."
Learned
Reviews
Taylor[121], reviewing potential medical uses in
1998, concluded: "Marijuana
shows clinical promise for... spasticity, multiple sclerosis...
As a medical drug, marijuana should be available for
patients who do not adequately respond to currently
available therapies."
In
2002 reviews, Grundy[122] reports "Cannabinoids
... provide symptomatic relief in experimental models
of chronic neurodegenerative diseases, such as multiple
sclerosis and Huntington's disease",
but cautioned "Our
understanding of cannabinoid neurobiology, however,
must improve if we are to effectively exploit this system
and take advantage of the numerous characteristics that
make this group of compounds potential neuroprotective
agents." Pertwee[123] notes "Clinical
evidence comes from trials, albeit with rather small
numbers of patients. These trials have shown that cannabis,
Delta(9)-tetrahydrocannabinol, and nabilone can produce
objective and/or subjective relief from spasticity,
pain, tremor, and nocturia in patients with multiple
sclerosis (8 trials)", noting "...experiments,
... with mice ... have provided strong evidence that
cannabinoid-induced reductions in tremor and spasticity
are mediated by cannabinoid receptors."
Pertwee & Ross[124] concluded "Potential
therapeutic uses of cannabinoid receptor agonists include
the management of multiple sclerosis/spinal cord injury,
pain, inflammatory disorders, glaucoma, bronchial asthma,
vasodilation that accompanies advanced cirrhosis, and
cancer."
In
a 2002 review Smith[125] concluded "There
is a large amount of evidence to support the view that
the psychoactive ingredient in cannabis, delta9-tetrahydrocannabinol
(delta9-THC), and cannabinoids in general, can reduce
muscle spasticity and pain under some circumstances.
Cannabinoid (CB1) receptors in the CNS appear to mediate
both of these effects and endogenous cannabinoids may
fulfil these functions to some extent under normal circumstances.
However, in the context of multiple sclerosis (MS),
it is still questionable whether cannabinoids are superior
to existing, conventional medications for the treatment
of spasticity and pain. ... Synthetic cannabinoids,
which target specific cannabinoid receptor subtypes
in specific parts of the CNS, are likely to be of more
therapeutic use than delta9-THC itself"
Contemporaneously, Pertwee[126] concluded
"There is a growing
amount of evidence to suggest that cannabis and individual
cannabinoids may be effective in suppressing certain
symptoms of multiple sclerosis and spinal cord injury,
including spasticity and pain.", noting
that "trials have shown that cannabis, Delta(9)-tetrahydrocannabinol,
and nabilone can produce objective and/or subjective
relief from spasticity, pain, tremor, and nocturia in
patients with multiple sclerosis (8 trials) or spinal
cord injury (1 trial). The clinical evidence is supported
by results from experiments with animal models of multiple
sclerosis. Some of these experiments, performed with
mice with chronic relapsing experimental allergic encephalomyelitis
(CREAE), have provided strong evidence that cannabinoid-induced
reductions in tremor and spasticity are mediated by
cannabinoid receptors, both CB(1) and CB(2)."
In 2005 Smith[127] qualified his skepticism, noting "The evidence for the therapeutic efficacy of cannabinoids
in the treatment of multiple sclerosis (MS) is increasing
but is not as yet convincing. Although several trials
have reported no significant effect, the majority of
the evidence which supports a beneficial effect on spasticity
and pain is based on subjective measurements in trials
where unblinding was likely to be a problem. The available
clinical trial data suggest that the adverse side effects
associated with using cannabis-based medicinal extracts
(CBMEs) are generally mild, such as dry mouth, dizziness,
somnolence, nausea and intoxication, and in no case
did toxicity develop."
Baker
& Pryce[128] noted "those
with multiple sclerosis, claim that it may offer benefit
in symptom control. Cannabis exerts many of its effects
because it taps into an endogenous cannabinoid system...
Cannabinoids provide a novel therapeutic target, not
only for controlling symptoms, but also slowing disease
progression through inhibition of neurodegeneration,
which is the cause of accumulating irreversible disability."
Pryce & Baker[129] concluded
"abundant experimental
data have reinforced the anecdotal claims of people
who perceive medicinal benefit from the currently illegal
consumption of cannabis. This, combined with data from
recent clinical trials, points to the prospect of cannabis
as a medication in the treatment of multiple sclerosis
and numerous other medical conditions."
Croxford
& Miller[130], reviewing early clinical trials, noted
"recent research
in animal models of multiple sclerosis has demonstrated
the efficacy of cannabinoids in controlling disease-induced
symptoms such as spasticity and tremor, as well as in
ameliorating the severity of clinical disease. However,
these initially promising results have not yet been
fully translated into the clinic. Although cannabinoid
treatment of multiple sclerosis symptoms has been shown
to be both well tolerated and effective in a number
of subjective tests in several small-scale clinical
trials, objective measures demonstrating the efficacy
of cannabinoids are still lacking."
Reviewing clinical trial and animal evidence, Schwarz
et al[131] concluded "Many
patients use cannabis to alleviate spasticity and pain.
Small series indicated positive effects, but randomized
trials were negative for spasticity. However, many patients
report subjective improvement under cannabis even if
their objective parameters remain unchanged"
Trebst & Sangel concluded "there
is reasonable evidence for the therapeutical employment
of cannabinoids in the treatment of MS related symptoms.
Furthermore, data are arising that cannabinoids have
immunomodulatory and neuroprotective properties. However,
results from clinical trials do not allow the recommendation
for the general use of cannabinoids in MS"
In
December 2005, Malfitano et al[132] summarised the state
of knowledge of the pharmacological, neuroanatomical
and biochemical mechanisms behind the role of cannabinoids
in MS thus "An
increasing body of evidence suggests that cannabinoids
have beneficial effects on the symptoms of multiple
sclerosis, including spasticity and pain. Endogenous
molecules with cannabinoid-like activity, such as the
"endocannabinoids", have been shown to mimic
the anti-inflammatory properties of cannabinoids through
the cannabinoid receptors. Several studies suggest that
cannabinoids and endocannabinoids may have a key role
in the pathogenesis and therapy of multiple sclerosis.
Indeed, they can down regulate the production of pathogenic
T helper 1-associated cytokines enhancing the production
of T helper 2-associated protective cytokines. A shift
towards T helper 2 has been associated with therapeutic
benefit in multiple sclerosis. In addition, cannabinoids
exert a neuromodulatory effect on neurotransmitters
and hormones involved in the neurodegenerative phase
of the disease. In vivo studies using mice with experimental
allergic encephalomyelitis, an animal model of multiple
sclerosis, suggest that the increase of the circulating
levels of endocannabinoids might have a therapeutic
effect, and that agonists of endocannabinoids with low
psychoactive effects could open new strategies for the
treatment of multiple sclerosis."
Pertwee[133] summarised "CB1
and/or CB2 receptor activation appears to ameliorate
inflammatory and neuropathic pain and certain multiple
sclerosis symptoms. This might be exploited clinically
by using CB1, CB2 or CB1/CB2 agonists, or inhibitors
of the membrane transport or catabolism of endocannabinoids
that are released in increased amounts, at least in
animal models of pain and multiple sclerosis."
McFarland et al[134][135] concluded
"augmentation
of cannabinergic tone might be therapeutically beneficial
in the treatment of multiple disease states such as
chronic pain, anxiety, multiple sclerosis, and neuropsychiatric
disorders"
Summary
- Cannabinoids and M.S.
Multiple
Sclerosis is a disease for which conventional medication
provides little benefit.
There
is a wealth of anecdotal evidence from MS patients reporting
dramatic improvement in symptoms following illicit use
of cannabis, from case histories and from surveys.
The
results of early small-scale clinical trials were mixed,
although more recent large scale clinical trials have
shown THC and cannabis extracts can improve, in some
cases dramatically, symptoms of MS such as pain, ataxia,
muscle spasm, spasticity, bladder dysfunction and tremor
in many (but not all) patients. Studies failing to find,
or finding non-significant effects, have tended to use
subtherapeutic doses.
Recent
animal research has indicated a direct receptor mediated
immunosuppressive effect on microglial cells in the
brain tissues which may delay or in some cases reverse
the neurodegenerative process in MS-like animal models.
This provides growing evidence that cannabinoids may
not only benefit the symptoms of MS, but may potentially
provide a treatment for the disease process itself,
offering unprecedented hope to sufferers of the disease.
The
House of Lords Science & Technology Committee recommended
in 1998 that clinical trials of cannabinoids in the
treatment of MS be undertaken as a matter of urgency,
and that pending the award of product licences, doctors
should be allowed to prescribe cannabis or cannabis
resin as an unlicensed medicine on a named-patient basis
for patients, including MS sufferers. In 2001, they
went further and recommended that cannabis preparations
be legalised for medical use. Since 1998 scientific
investigation of the effects and causes of cannabinoid
action on MS and symptoms has exploded, with the vast
majority of studies and reviewers showing a potential
therapeutic benefit. However the Medicines Control Agency
(MCA) and National Institute for Clinical Excellence
(NICE) have yet to licence cannabis, THC or Sativex
for routine medical prescription.
Clinical
trials of the extract Sativex containing roughly equal quantities
of THC and CBD have found the combination more effective
than THC alone, largely due to the increased amount
of THC which can be administered before patients develop
a åhigh", but also due to the anti-inflammatory
effects of CBD.
M.J.Atha
© IDMU Ltd - September 2006
References
[1]
Macpherson G. (ed) [1995] Black"s Medical Dictionary
38th Edition. London: A&C Black pp332-333
[2]
House of Lords [1998] op cit para 5.19
[3]
Grinspoon L & Bakalar JB (1997) Marijuana - Forbidden
Medicine (2nd Ed). Yale University Press
[5]
Consroe P, Musty R, Rein J, Tillery W, Pertwee R (1997)
The perceived effects of smoked cannabis on patients
with multiple sclerosis. Eur Neurol 38(1):44-8
[6]
Schnelle M, Grotenhermen F, Reif M, Gorter RW (1999)
[Results of a standardized survey on the medical use
of cannabis products in the German-speaking area]. [Article
in German] Forsch Komplementarmed 6 Suppl 3:28-36
[7]
Mechoulam R (1999) Recent advantages in cannabinoid
research. Forsch Komplementarmed 6 Suppl 3:16-20
[8]
Somerset M, Campbell R, Sharp DJ, Peters TJ [2000] What
do people with MS want and expect from health-care services?
Health Expect 4(1):29-37
[9]
Page SA, Verhoef MJ, Stebbins RA, Metz LM, Levy JC.
[2003] Cannabis use as described by people with multiple
sclerosis. Can J Neurol Sci. 30(3):201-5.
[10]
Amtmann D, Weydt P, Johnson KL, Jensen MP, Carter GT.
[2004] Survey of cannabis use in patients with amyotrophic
lateral sclerosis. Am J Hosp Palliat Care. 21(2):95-104.
[11]
Simmons RD, Ponsonby AL, van der Mei IA, Sheridan P.
[2004] What affects your MS? Responses to an anonymous,
Internet-based epidemiological survey. Mult Scler. 2004
Apr;10(2):202-11.
[12]
Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. [2004]
Patterns of cannabis use among patients with multiple
sclerosis. Neurology. 62(11):2098-100.
[13]
Ware MA, Adams H, Guy GW. [2005] The medicinal use of
cannabis in the UK: results of a nationwide survey.
Int J Clin Pract. 59(3):291-5.
[14]
Erkens JA, Janse AF, Herings RM. [2005] Limited use
of medicinal cannabis but for labeled indications after
legalization. Pharmacoepidemiol Drug Saf. 14(11):821-2
[15]
LymanWD, Sonett JR, Brosnan CF, Elkin R & Bornstein
MB (1989) Delta-9-tetrahydrocannabinol: an novel treatment
for Experimental Autoimmune Encephalomyelitis. Journal
of Neuroimmunology 23 pp73-81
[16]
Wirguin I, Mechoulam R, Breuer A, Schezen E, Weidenfeld
J & Brenner T (1994) Suppression of Experimental
Autoimmune Encephalomyelitis by cannabinoids. Immunopharmacology
28(3) pp209-214
[17]
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG,
Huffman JW, Layward L (2000) Cannabinoids control spasticity
and tremor in a multiple sclerosis model. Nature 404(6773):84-7
[18]
Achiron A, Miron S, Lavie V, Margalit R, Biegon A (2000)
Dexanabinol (HU-211) effect on experimental autoimmune
encephalomyelitis: implications for the treatment of
acute relapses of multiple sclerosis. J Neuroimmunol
102(1):26-31
[19]
Pop E. [2000] Dexanabinol Pharmos. Curr Opin Investig
Drugs 1(4):494-503
[20]
Berrendero F, Sanchez A, Cabranes A, Puerta C, Ramos
JA, Garcia-Merino A, Fernandez-Ruiz J. [2001] Changes
in cannabinoid CB(1) receptors in striatal and cortical
regions of rats with experimental allergic encephalomyelitis,
an animal model of multiple sclerosis. Synapse 41(3):195-202
[21]
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG,
Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno
T, Di Marzo V [2001] Endocannabinoids control spasticity
in a multiple sclerosis model. FASEB J 15(2):300-2
[22]
Di Marzo V, Bifulco M, De Petrocellis L (2000) Endocannabinoids
and multiple sclerosis: a blessing from the 'inner bliss'?
Trends Pharmacol Sci 21(6):195-7
[23]
Pop E (2000) Dexanabinol Pharmos. Curr Opin Investig
Drugs 1(4):494-503
[24]
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG,
Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno
T, Di Marzo V (2001) Endocannabinoids control spasticity
in a multiple sclerosis model. FASEB J 15(2):300-2
[25]
Pertwee RG (1999) Cannabis and cannabinoids: pharmacology
and rationale for clinical use. Forsch Komplementarmed
6 Suppl 3:12-5
[26]
Pertwee RG (1999) Pharmacology of cannabinoid receptor
ligands. Curr Med Chem 6(8):635-64
[27]
Killestein J, Nelemans SA (1997) [Therapeutic applications
and biomedical effects of cannabinoids; pharmacological
starting points]. [Article in Dutch] Ned Tijdschr Geneeskd
141(35):1689-93
[28]
Klein TW, Newton CA, Friedman H [2001] Cannabinoids
and the immune system. Pain Res Manag 6(2):95-101
[29]
Lambert DM, Vandevoorde S, Jonsson KO, Fowler CJ. [2002]
The palmitoylethanolamide family: a new class of anti-inflammatory
agents? Curr Med Chem 9(6):663-74
[30]
Wilkinson JD, Whalley BJ, Baker D, Pryce G, Constanti
A, Gibbons S, Williamson EM. [2003] Medicinal cannabis:
is delta9-tetrahydrocannabinol necessary for all its
effects? J Pharm Pharmacol. 55(12):1687-94.
[31]
Ni X, Geller EB, Eppihimer MJ, Eisenstein TK, Adler
MW, Tuma RF. [2004] Win 55212-2, a cannabinoid receptor
agonist, attenuates leukocyte/endothelial interactions
in an experimental autoimmune encephalomyelitis model.
Mult Scler. 10(2):158-64
[32]
Raman C, McAllister SD, Rizvi G, Patel SG, Moore DH,
Abood ME. [2004] Amyotrophic lateral sclerosis: delayed
disease progression in mice by treatment with a cannabinoid.
Amyotroph Lateral Scler Other Motor Neuron Disord. 5(1):33-9.
[33]
Mestre L, Correa F, Arevalo-Martin A, Molina-Holgado
E, Valenti M, Ortar G, Di Marzo V, Guaza C. [2005] Pharmacological
modulation of the endocannabinoid system in a viral
model of multiple sclerosis. J Neurochem. 92(6):1327-39.
[34]
Weydt P, Hong S, Witting A, Moller T, Stella N, Kliot
M. [2005] Cannabinol delays symptom onset in SOD1 (G93A)
transgenic mice without affecting survival. Amyotroph
Lateral Scler Other Motor Neuron Disord. 6(3):182-4.
[35]
Ortega-Gutierrez S, Molina-Holgado E, Arevalo-Martin
A, Correa F, Viso A, Lopez-Rodriguez ML, Di Marzo V,
Guaza C. [2005] Activation of the endocannabinoid system
as therapeutic approach in a murine model of multiple
sclerosis. FASEB J 19(10):1338-40
[36]
de Lago E, Fernandez-Ruiz J, Ortega-Gutierrez S, Cabranes
A, Pryce G, Baker D, Lopez-Rodriguez M, Ramos JA. [2006]
UCM707, an inhibitor of the anandamide uptake, behaves
as a symptom control agent in models of Huntington's
disease and multiple sclerosis, but fails to delay/arrest
the progression of different motor-related disorders.
Eur Neuropsychopharmacol. 16(1):7-18.
[37]
Arevalo-Martin A, Vela JM, Molina-Holgado E, Borrell
J, Guaza C. [2003] Therapeutic action of cannabinoids
in a murine model of multiple sclerosis. J Neurosci.
23(7):2511-6.
[38]
Croxford JL, Miller SD. [2003] Immunoregulation of a
viral model of multiple sclerosis using the synthetic
cannabinoid R+WIN55,212. J Clin Invest. 111(8):1231-40.
[39]
Cabranes A, Pryce G, Baker D, Fernandez-Ruiz J. [2006]
Changes in CB(1) receptors in motor-related brain structures
of chronic relapsing experimental allergic encephalomyelitis
mice. Brain Res. 1107(1):199-205
[40]
Clark PA (2000) The ethics of medical marijuana: government
restrictions vs. medical necessity. J Public Health
Policy 21(1):40-60
[41]
Rosenthal MS, Kleber HD (1999) Making sense of medical
marijuana. Proc Assoc Am Physicians 111(2):159-65
[42]
Masood E (1998) Cannabis laws 'threaten validity of
trials'. Nature 396(6708):206
[43]
Robson P. [2005] Human studies of cannabinoids and medicinal
cannabis. Handb Exp Pharmacol. (168):719-56.
[44]
Petro DJ, Ellenberger C Jr (1981) Treatment of human
spasticity with delta 9-tetrahydrocannabinol. J Clin
Pharmacol 21(8-9 Suppl):413S-416S
[45]
Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW,
Myers LW (1987) Delta-9-THC in the treatment of spasticity
associated with multiple sclerosis. Adv Alcohol Subst
Abuse 7(1):39-50
[46]
Clifford DB (1983) Tetrahydrocannabinol for tremor in
multiple sclerosis. Ann Neurol 13(6):669-71
[47]
Schon F, Hart PE, Hodgson TL, Pambakian AL, Ruprah M,
Williamson EM, Kennard C (1999) Suppression of pendular
nystagmus by smoking cannabis in a patient with multiple
sclerosis. Neurology 53(9):2209-10
[48]
Dell'Osso LF (2000) Suppression of pendular nystagmus
by smoking cannabis in a patient with multiple sclerosis.
Neurology 54(11):2190-3
[49]
Brenneisen R, Egli A, Elsohly MA, Henn V, Spiess Y (1996)
The effect of orally and rectally administered delta
9-tetrahydrocannabinol on spasticity: a pilot study
with 2 patients. Int J Clin Pharmacol Ther 34(10):446-52
[50]
Maurer M, Henn V, Dittrich A, Hofmann A (1990) Delta-9-tetrahydrocannabinol
shows antispastic and analgesic effects in a single
case double-blind trial. Eur Arch Psychiatry Neurol
Sci 1990;240(1):1-4
[51]
Consroe P, Sandyk R, Snider SR (1986) Open label evaluation
of cannabidiol in dystonic movement disorders. Int J
Neurosci 30(4):277-82
[52]
Malec J, Harvey RF, Cayner JJ (1982) Cannabis effect
on spasticity in spinal cord injury. Arch Phys Med Rehabil
63(3):116-8
[53]
Petro DJ (1980) Marihuana as a therapeutic agent for
muscle spasm or spasticity. Psychosomatics 21(1):81,
85
[54]
Check WA (1979) Marijuana may lessen spasticity of MS.
JAMA 241(23):2476
[55]
Martyn CN, Illis LS, Thom J (1995) Nabilone in the treatment
of multiple sclerosis. Lancet 345(8949):579
[56]
Meinck HM, Schonle PW, Conrad B (1989) Effect of cannabinoids
on spasticity and ataxia in multiple sclerosis. J Neurol
236(2):120-2
[57]
Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van
Loenen AC, Staats PG, Gorter RW, Uitdehaag BM, Polman
CH. [2002] Safety, tolerability, and efficacy of orally
administered cannabinoids in MS. Neurology 58(9):1404-7
[58]
Thompson AJ, Baker D. [2002] Cannabinoids in MS: potentially
useful but not just yet! Neurology58(9):1323-4
[59]
Smith PF. [2002] Cannabinoids in the treatment of pain
and spasticity in multiple sclerosis. Curr Opin Investig
Drugs 3(6):859-64
[60]
Lorenzo Fernandez P (2000) [No title available].[Article
in Spanish] An R Acad Nac Med (Madr) 117(3):595-605;
discussion 616-24
[61]
Clark PA (2000) The ethics of medical marijuana: government
restrictions vs. medical necessity. J Public Health
Policy 2000;21(1):40-60
[62]
Williamson EM, Evans FJ (2000) Cannabinoids in clinical
practice. Drugs 60(6):1303-14
[63]
Mechoulam R, Hanu L. [2001] The cannabinoids: An overview.
Therapeutic implications in vomiting and nausea after
cancer chemotherapy, in appetite promotion, in multiple
sclerosis and in neuroprotection. Pain Res Manag 6(2):67-73
[64]
Schlicker E, Kathmann M. [2001] Modulation of transmitter
release via presynaptic cannabinoid receptors. Trends
Pharmacol Sci 22(11):565-72
[65]
Carter GT, Rosen BS [2001] Marijuana in the management
of amyotrophic lateral sclerosis. Am J Hosp Palliat
Care 18(4):264-70
[66]
Guzman M, Sanchez C, Galve-Roperh I. [2001] Control
of the cell survival/death decision by cannabinoids.
J Mol Med 78(11):613-25
[67]
Petro DJ, Ellenberger C Jr [1981] Treatment of human
spasticity with delta 9-tetrahydrocannabinol. J Clin
Pharmacol 21(8-9 Suppl):413S-416S
[68]
Malec J, Harvey RF, Cayner JJ. [1982] Cannabis effect
on spasticity in spinal cord injury. Arch Phys Med Rehabil
63(3):116-8
[69]
Pertwee R. [2002] Cannabinoids and multiple sclerosis.
Pharmacol Ther 2002 Aug;95(2):165
[70]
Pertwee RG, Ross RA. [2002] Cannabinoid receptors and
their ligands. Prostaglandins Leukot Essent Fatty Acids
66(2-3):101-21
[71]
Brooks JW, Pryce G, Bisogno T, Jaggar SI, Hankey DJ,
Brown P, Bridges D, Ledent C, Bifulco M, Rice AS, Di
Marzo V, Baker D. [2002] Arvanil-induced inhibition
of spasticity and persistent pain: evidence for therapeutic
sites of action different from the vanilloid VR1 receptor
and cannabinoid CB(1)/CB(2) receptors. Eur J Pharmacol
439(1-3):83-92
[72]
Smith PF. [2002] Cannabinoids in the treatment of pain
and spasticity in multiple sclerosis. Curr Opin Investig
Drugs 3(6):859-64
[73]
Kalant H. [2001] Medicinal use of cannabis: history
and current status. Pain Res Manag 6(2):80-91
[74]
Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van
Loenen AC, Staats PG, Gorter RW, Uitdehaag BM, Polman
CH. [2002] Safety, tolerability, and efficacy of orally
administered cannabinoids in MS. Neurology 58(9):1404-7
[75]
Thompson AJ, Baker D. [2002] Cannabinoids in MS: potentially
useful but not just yet! Neurology 58(9):1323-4
[78]
Svendsen KB, Jensen TS, Bach FW. [2004] Does the cannabinoid
dronabinol reduce central pain in multiple sclerosis?
Randomised double blind placebo controlled crossover
trial. BMJ. 329(7460):253
[79]
Svendsen KB, Jensen TS, Bach FW. [2005] [Effect of the
synthetic cannabinoid dronabinol on central pain in
patients with multiple sclerosis--secondary publication]
[Article in Danish] Ugeskr Laeger. 167(25-31):2772-4.
[80]
Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F,
Gattlen B, Hagen U, Schnelle M, Reif M. [2004] Efficacy,
safety and tolerability of an orally administered cannabis
extract in the treatment of spasticity in patients with
multiple sclerosis: a randomized, double-blind, placebo-controlled,
crossover study. Mult Scler. 10(4):417-24.
[81]
Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley
KJ, Fowler CJ. [2004] An open-label pilot study of cannabis-based
extracts for bladder dysfunction in advanced multiple
sclerosis. Mult Scler. 10(4):425-33.
[82]
Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE,
Wright D, Zajicek J. [2006] The effect of cannabis on
urge incontinence in patients with multiple sclerosis:
a multicentre, randomised placebo-controlled trial (CAMS-LUTS).
Int Urogynecol J Pelvic Floor Dysfunct. 2006 Mar 22;
[Epub ahead of print]
[83]
Wade DT, Makela P, Robson P, House H, Bateman C. [2004]
Do cannabis-based medicinal extracts have general or
specific effects on symptoms in multiple sclerosis?
A double-blind, randomized, placebo-controlled study
on 160 patients. Mult Scler. 10(4):434-41.
[84]
Rog DJ, Nurmikko TJ, Friede T, Young CA. [2005] Randomized,
controlled trial of cannabis-based medicine in central
pain in multiple sclerosis. Neurology. 65(6):812-9.
[85]
Teare L, Zajicek J. [2005] The use of cannabinoids in
multiple sclerosis. Expert Opin Investig Drugs. 14(7):859-69.
[86]
Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram
WM, Reilly SM, Nunn AJ, Teare LJ, Fox PJ, Thompson AJ.
[2005] Cannabinoids in multiple sclerosis (CAMS) study:
safety and efficacy data for 12 months follow up. J
Neurol Neurosurg Psychiatry. 76(12):1664-9.
[87]
Azad SC, Rammes G. [2005] Cannabinoids in anaesthesia
and pain therapy. Curr Opin Anaesthesiol. 18(4):424-7.
[88]
Solaro C. [2006] Epidemiology and treatment of pain
in multiple sclerosis subjects. Neurol Sci. 27 Suppl
4:s291-3.
[89]
Wade DT, Robson P, House H, Makela P, Aram J.[2003]
A preliminary controlled study to determine whether
whole-plant cannabis extracts can improve intractable
neurogenic symptoms. Clin Rehabil. 17(1):21-9.
[90]
Fox P, Bain PG, Glickman S, Carroll C, Zajicek J. [2004]
The effect of cannabis on tremor in patients with multiple
sclerosis. Neurology. 62(7):1105-9.
[91]
Notcutt W, Price M, Miller R, Newport S, Phillips C,
Simmons S, Sansom C. [2004] Initial experiences with
medicinal extracts of cannabis for chronic pain: results
from 34 'N of 1' studies. Anaesthesia. 59(5):440-52
[92]
[No authors listed] [2003] Cannabis-based medicines--GW
pharmaceuticals: high CBD, high THC, medicinal cannabis--GW
pharmaceuticals, THC:CBD. Drugs R D. 4(5):306-9.
[93]
Corey S. [2005] Recent developments in the therapeutic
potential of cannabinoids. P R Health Sci J. 24(1):19-26.
[94]
Smith PF. [2004] Medicinal cannabis extracts for the
treatment of multiple sclerosis. Curr Opin Investig
Drugs. 5(7):727-30.
[95]
Fernandez-Ruiz J, Lastres-Becker I, Cabranes A, Gonzalez
S, Ramos JA. [2002] Endocannabinoids and basal ganglia
functionality. Prostaglandins Leukot Essent Fatty Acids.
66(2-3):257-67.
[96]
Shakespeare DT, Boggild M, Young C. [2003] Anti-spasticity
agents for multiple sclerosis. Cochrane Database Syst
Rev. (4):CD001332
[97]
Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn
A, Thompson A; UK MS Research Group. [2003] Cannabinoids
for treatment of spasticity and other symptoms related
to multiple sclerosis (CAMS study): multicentre randomised
placebo-controlled trial. Lancet. 362(9395):1517-26.
[98]
Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van
Loenen AC, Staats PG, Gorter RW, Uitdehaag BM, Polman
CH. [2002] Safety, tolerability, and efficacy of orally
administered cannabinoids in MS. Neurology. 58(9):1404-7.
[99]
Killestein J, Hoogervorst EL, Reif M, Blauw B, Smits
M, Uitdehaag BM, Nagelkerken L, Polman CH. [2003] Immunomodulatory
effects of orally administered cannabinoids in multiple
sclerosis. J Neuroimmunol. 137(1-2):140-3.
[100]
Killestein J, Uitdehaag BM, Polman CH. [2004] Cannabinoids
in multiple sclerosis: do they have a therapeutic role?
Drugs. 64(1):1-11.
[101]
Killestein J, Bet PM, van Loenen AC, Polman CH. [2004]
[Medicinal cannabis for diseases of the nervous system:
no convincing evidence of effectiveness] [Article in
Dutch] Ned Tijdschr Geneeskd. 148(48):2374-8.
[102]
Russo E, Guy GW. [2006] A tale of two cannabinoids:
the therapeutic rationale for combining tetrahydrocannabinol
and cannabidiol. Med Hypotheses. 66(2):234-46.
[103]
Perez J. [2006] Combined cannabinoid therapy via an
oromucosal spray. Drugs Today (Barc). 42(8):495-503.
[104]
Molina-Holgado F, Molina-Holgado E, Guaza C (1998) The
endogenous cannabinoid anandamide potentiates interleukin-6
production by astrocytes infected with Theiler's murine
encephalomyelitis virus by a receptor-mediated pathway.
FEBS Lett 14;433(1-2):139-42
[105]
van Oosten BW, Killestein J, Mathus-Vliegen EM, Polman
CH. [2004] Multiple sclerosis following treatment with
a cannabinoid receptor-1 antagonist. Mult Scler. 10(3):330-1.
[106]
Jackson SJ, Pryce G, Diemel LT, Cuzner ML, Baker D.
[2005] Cannabinoid-receptor 1 null mice are susceptible
to neurofilament damage and caspase 3 activation. Neuroscience.
134(1):261-8.
[107]
Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL,
Pocock JM, Ledent C, Petzold A, Thompson AJ, Giovannoni
G, Cuzner ML, Baker D. [2003] Cannabinoids inhibit neurodegeneration
in models of multiple sclerosis. Brain. 126(Pt 10):2191-202.
[108]
Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang
W, Nithipatikom K, Pfister SL, Campbell WB, Hillard
CJ. [2004] Cultured rat microglial cells synthesize
the endocannabinoid 2-arachidonylglycerol, which increases
proliferation via a CB2 receptor-dependent mechanism.
Mol Pharmacol. 65(4):999-1007.
[109]
Kim K, Moore DH, Makriyannis A, Abood ME. [2006] AM1241,
a cannabinoid CB2 receptor selective compound, delays
disease progression in a mouse model of amyotrophic
lateral sclerosis. Eur J Pharmacol. 542(1-3):100-5.
[110]
Stella N. [2004] Cannabinoid signaling in glial cells.
Glia. 48(4):267-77.
[111]
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein
T, Fernandez F, Tan J, Shytle RD. [2005] Stimulation
of cannabinoid receptor 2 (CB2) suppresses microglial
activation. J Neuroinflammation. 12;2:29.
[112]
Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt
PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R,
Nitsch R, Ullrich O. [2006] The endocannabinoid anandamide
protects neurons during CNS inflammation by induction
of MKP-1 in microglial cells. Neuron. 49(1):67-79.
[113]
Tagliaferro P, Javier Ramos A, Onaivi ES, Evrard SG,
Lujilde J, Brusco A. [2006] Neuronal cytoskeleton and
synaptic densities are altered after a chronic treatment
with the cannabinoid receptor agonist WIN 55,212-2.
Brain Res. 1085(1):163-76.
[114]
Fujiwara M, Egashira N. [2004] New perspectives in the
studies on endocannabinoid and cannabis: abnormal behaviors
associate with CB1 cannabinoid receptor and development
of therapeutic application. J Pharmacol Sci. 96(4):362-6.
[115]
Yang C, Hader W, Zhang X. [2006] Therapeutic action
of cannabinoid on axonal injury induced by peroxynitrite.
Brain Res. 1076(1):238-42.
[116]
Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor
A, Bountra C, Banati RR, Anand P. [2006] COX-2, CB2
and P2X7-immunoreactivities are increased in activated
microglial cells/macrophages of multiple sclerosis and
amyotrophic lateral sclerosis spinal cord. BMC Neurol.
2;6:12.
[117]
Witting A, Chen L, Cudaback E, Straiker A, Walter L,
Rickman B, Moller T, Brosnan C, Stella N. [2006] Experimental
autoimmune encephalomyelitis disrupts endocannabinoid-mediated
neuroprotection. Proc Natl Acad Sci U S A. 103(16):6362-7
[118]
Bilsland LG, Dick JR, Pryce G, Petrosino S, Di Marzo
V, Baker D, Greensmith L. [2006] Increasing cannabinoid
levels by pharmacological and genetic manipulation delay
disease progression in SOD1 mice. FASEB J. 20(7):1003-5.
[119]
Jackson SJ, Baker D, Cuzner ML, Diemel LT. [2004] Cannabinoid-mediated
neuroprotection following interferon-gamma treatment
in a three-dimensional mouse brain aggregate cell culture.
Eur J Neurosci.20(9):2267-75.
[120]
Jackson SJ, Diemel LT, Pryce G, Baker D. [2005] Cannabinoids
and neuroprotection in CNS inflammatory disease. J Neurol
Sci.233(1-2):21-5.
[121]
Taylor HG (1998) Analysis of the medical use of marijuana
and its societal implications. J Am Pharm Assoc (Wash)
38(2):220-7
[122]
Grundy RI. [2002] The therapeutic potential of the cannabinoids
in neuroprotection. Expert Opin Investig Drugs 11(10):1365-74
[123]
Pertwee R. [2002] Cannabinoids and multiple sclerosis
Pharmacol Ther 295(2):165
[124]
Pertwee RG, Ross RA. [2002] Cannabinoid receptors and
their ligands. Prostaglandins Leukot Essent Fatty Acids
66(2-3):101-21
[125]
Smith PF. [2002] Cannabinoids in the treatment of pain
and spasticity in multiple sclerosis. Curr Opin Investig
Drugs. 3(6):859-64.
[126]
Pertwee RG. [2002] Cannabinoids and multiple sclerosis.Pharmacol
Ther. 2002 Aug;95(2):165-74.
[127]
Smith PF. [2005] The safety of cannabinoids for the
treatment of multiple sclerosis. Expert Opin Drug Saf.
4(3):443-56.
[128]
Baker D, Pryce G. [2003] The therapeutic potential of
cannabis in multiple sclerosis. Expert Opin Investig
Drugs. 12(4):561-7.
[129]
Pryce G, Baker D. [2005] Emerging properties of cannabinoid
medicines in management of multiple sclerosis. Trends
Neurosci. 28(5):272-6.
[130]
Croxford JL, Miller SD. [2004] Towards cannabis and
cannabinoid treatment of multiple sclerosis. Drugs Today
(Barc). 40(8):663-76.
[131]
Schwarz S, Leweling H, Meinck HM. [2005] [Alternative
and complementary therapies in multiple sclerosis] [Article
in German] Fortschr Neurol Psychiatr. 73(8):451-62
[132]
Malfitano AM, Matarese G, Bifulco M. [2005] From cannabis
to endocannabinoids in multiple sclerosis: a paradigm
of central nervous system autoimmune diseases. Curr
Drug Targets CNS Neurol Disord. 4(6):667-75.
[133]
Pertwee RG. [2006] The pharmacology of cannabinoid receptors
and their ligands: an overview. Int J Obes (Lond). 30
Suppl 1:S13-8.
[135]
McFarland MJ, Terebova EA, Barker EL. [2006] Detergent-resistant
membrane microdomains in the disposition of the lipid
signaling molecule anandamide. AAPS J. 8(1):E95-100.
|
All contents of this web
site & any links to other sites etc, is for educational
& research purposes. IDMU at no time seeks to encourage
illegal activities. All sections of this site and its contents
are protected under copyright laws. © IDMU
Ltd 1994 - 2008
|
|
|
|